ICH Q1B 光稳定性试验(中英文)
INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE
ICH HARMONISED TRIPARTITE GUIDELINE
STABILITY TESTING: 稳定性试验
PHOTOSTABILITY TESTING OF NEW DRUG SUBSTANCES AND PRODUCTS
新原料药和新制剂药物的光稳定性测试
Q1B
Current Step 4 version
现行版本第4步骤
dated 6 November 1996
1996年11月6日
This Guideline has been developed by the appropriate ICH Expert Working Group and has been subject to consultation by the regulatory parties, in accordance with the ICH Process. At Step 4 of the Process the final draft is recommended for adoption to the regulatory bodies of the European Union, Japan and USA.
Q1B Document History 文件历史
|
First Codification |
History |
Date |
New Codification November 2005 |
|
Q1B |
Approval by the Steering Committee under Step 2 and release for public consultation |
28 November 1995 |
Q1B |
Current Step 4 version
|
Q1B |
Approval by the Steering Committee under Step 4 and recommendation for adoption to the three ICH regulatory bodies |
6 November 1996 |
Q1B |
STABILITY TESTING: PHOTOSTABILITY TESTING OF NEW DRUG SUBSTANCES AND PRODUCTS
新原料药和新制剂药物的光稳定性测试
ICH Harmonised Tripartite Guideline
Having reached Step 4 of the ICH Process at the ICH Steering Committee meeting on 6 November 1996, this guideline is recommended for adoption to the three regulatory parties to ICH
TABLE OF CONTENTS 目录
1. General 概述......................................................................................................................1
A. Preamble 前言.............................................................................................................1
B. Light Sources 光源......................................................................................................2
C. Procedure 程序............................................................................................................2
2. Drug Substance 原料药.......................................................................................................4
A. Presentation of Samples 样品放置.....................................................................................4
B. Analysis of Samples 样品分析............................................................................................5
C. Judgement of Results 结果判断.........................................................................................5
3. Drug Product 制剂...........................................................................................................5
A. Presentation of Samples 样品放置.....................................................................................6
B. Analysis of Samples 样品分析............................................................................................6
C. Judgement of Results 结果判断.........................................................................................6
4. Annex 附件.........................................................................................................................7
A. Quinine Chemical Actinometry 奎宁化学计量测试......................................................7
5. Glossary 术语.....................................................................................................................8
6. References 参考文献................................................................................................................8
STABILITY TESTING: PHOTOSTABILITY TESTING OF NEW DRUG SUBSTANCES AND PRODUCTS
Q1B 新原料药和新制剂药物的光稳定性测试
1. GENERAL概述
The ICH Harmonized Tripartite Guideline covering the Stability Testing of New Drug Substances and Products (hereafter referred to as the Parent Guideline) notes that light testing should be an integral part of stress testing. This document is an annex to the Parent Guideline and addresses the recommendations for photostability testing.
新原料药和新药制剂的ICH稳定性试验指导原则(以下称总指导原则)指出光照试验是强力破坏试验中的重要组成部分。本文是总指导原则的附加说明,提出了光稳定性试验的一些建议。
A. Preamble前言
The intrinsic photostability characteristics of new drug substances and products should be evaluated to demonstrate that, as appropriate, light exposure does not result in unacceptable change. Normally, photostability testing is carried out on a single batch of material selected as described under Selection of Batches in the Parent Guideline. Under some circumstances these studies should be repeated if certain variations and changes are made to the product (e.g., formulation, packaging). Whether these studies should be repeated depends on the photostability characteristics determined at the time of initial filing and the type of variation and/or change made.
新原料药和新药制剂应经过适当的光稳定特征考察,证明其本身的光稳定性,即光照不能引起不可接受的变化。按照总指导原则中“批号选择”,光稳定性试验只须选做一批样品。在某些情况下如当这个产品发生变更或变化时(如处方、包装),这些研究应再重复进行。这些研究是否必须重复进行取决于初次申报文件中所测定的该物质的光稳定特征及变更或变化的类型。
The guideline primarily addresses the generation of photostability information for submission in Registration Applications for new molecular entities and associated drug products. The guideline does not cover the photostability of drugs after administration (i.e. under conditions of use) and those applications not covered by the Parent Guideline. Alternative approaches may be used if they are scientifically sound and justification is provided.
本指导原则主要阐述注册申报新化合物及其制剂时所需报送的光稳定性试验资料,不包括给药后(即使用中的)的光稳定性试验和总指导原则中未包括其用法的药物的申报内容。如果有科学合理的其他替代方法也可采用。
A systematic approach to photostability testing is recommended covering, as appropriate, studies such as:
建议光稳定性试验研究包括:
i) Tests on the drug substance;
I. 原料药光稳定性测试
ii) Tests on the exposed drug product outside of the immediate pack; and if necessary ;
II. 对除去内包装的制剂试验(如需要);
iii) Tests on the drug product in the immediate pack; and if necessary ;
III. 对除去外包装的制剂试验(如需要);
iv) Tests on the drug product in the marketing pack.
IV. 上市包装的制剂试验。
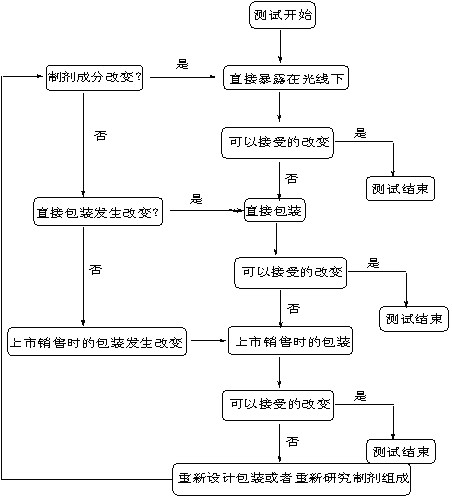
The extent of drug product testing should be established by assessing whether or not acceptable change has occurred at the end of the light exposure testing as described in the Decision Flow Chart for Photostability Testing of Drug Products. Acceptable change is change within limits justified by the applicant. The formal labeling requirements for photolabile drug substances and drug products are established by national/regional requirements.
按判断图指示进行药物光稳定性试验,可根据到哪一步发生了可以接受的变化来决定试验到哪一步即可停止,“可接受的变化”是指经申报者论证合理的限度内的变化。光敏性药物和制剂是否要在标签上标记要求,由国家和地区确定。
B. Light Sources 光源
The light sources desc ribed below may be used for photostability testing. The applicant should either maintain an appropriate control of temperature to minimize the effect of localized temperature changes or include a dark control in the same environment unless otherwise justified. For both options 1 and 2, a pharmaceutical manufacturer/applicant may rely on the spectral distribution specification of the light source manufacturer.
以下描述的光源可以用于光稳定性测试。申报者应该对温度进行适当的控制,以降低温度对该检测的影响或者是在相同的环境下进行暗度控制(避光对照),如有另有说明者除外。不管选择1还是2,药剂生产者或者申报者均可以采用光源生产商所给定的光谱分布规格数据。
Option 1
Any light source that is designed to produce an output similar to the D65/ID65 emission standard such as an artificial daylight fluorescent lamp combining visible and ultraviolet (UV) outputs, xenon, or metal halide lamp. D65 is the internationally recognized standard for outdoor daylight as defined in ISO 10977 (1993). ID65 is the equivalent indoor indirect daylight standard. For a light source emitting significant radiation below 320 nm, an appropriate filter(s) may be fitted to eliminate such radiation.
选择1:
采用任何输出相似于D65/ID65 发射标淮的光源,如具有可见光和紫外光输出的人造日光荧光灯、 氙灯或金属卤化物灯。D65 是国际上认可的室外日光标准,在1993年的ISO10977中对此进行了规定,ID65是相当于室内间接日光光照的标准。光源发射光低于320nm时,应当配备适当的装置去滤除此光。
Option 2 选择2
For option 2 the same sample should be exposed to both the cool white fluorescent and near ultraviolet lamp.
对于本选择,应使用相同的样品同时采用于冷白荧光灯和近紫外荧光灯照射。
1. A cool white fluorescent lamp designed to produce an output similar to that specified in ISO 10977(1993) ; and
冷白荧光灯应具有与ISO10977(1993)中规定的输出功率
2. A near UV fluorescent lamp having a spectral distribution from 320 nm to 400 nm with a maximum energy emission between 350 nm and 370 nm; a significant proportion of UV should be in both bands of 320 to 360 nm and 360 to 400 nm.
近紫外荧光灯可以发射的光线在320~400nm,在350~370nm有一个最大的能量发射;紫外线的重要组成部分应当是在320~360nm,和360~400nm的范围内
C. Procedure规程
For confirmatory studies, samples should be exposed to light providing an overall illumination of not less than 1.2 million lux hours and an integrated near ultraviolet energy of not less than 200 watt hours/square meter to allow direct comparisons to be made between the drug substance and drug product.
在确认研究中,样品应暴露在总照度不低于1.2×106Lux.hr,近紫外能量不低于200w.hr/m2,直接在药物和制剂之间进行比较。
Samples may be exposed side-by-side with a validated chemical actinometric system to ensure the specified light exposure is obtained, or for the appropriate duration of time when conditions have been monitored using calibrated radiometers/lux meters. An example of an actinometric procedure is provided in the Annex.
样品可与经论证过的光化强度系统并排暴露于有效的光化强度下,以确保获得指定的光暴露,或在用经校正的测光仪或照度仪监测的条件下,持续相当的时间。附录中提供了光化强度测定方法的例子。
If protected samples (e.g., wrapped in aluminum foil) are used as dark controls to evaluate the contribution of thermally induced change to the total observed change, these should be placed alongside the authentic sample.
若用遮光对照样品(如用铝箔包装)作为暗度控制来考察由热引起的变化对总变化的影响,应使其与真实样品并排放置。
注解:Lux:勒克斯,亮度单位,在英国叫做尺光度,在欧洲叫做lux,具体来说1勒克斯相当于一只蜡烛从1米外投射在一平方米的表面上的光的数量。
DECISION FLOW CHART FOR PHOTOSTABILITY TESTING OF DRUG PRODUCTS

药物光稳定性测试流程示意图
2. DRUG SUBSTANCE 原料药
For drug substances, photostability testing should consist of two parts: forced degradation testing and confirmatory testing.
对于原料药,光稳定性测试包括两个方面的内容:强制的降解试验和确认性试验
The purpose of forced degradation testing studies is to evaluate the overall photosensitivity of the material for method development purposes and/or degradation pathway elucidation. This testing may involve the drug substance alone and/or in simple solutions/suspensions to validate the analytical procedures. In these studies, the samples should be in chemically inert and transparent containers. In these forced degradation studies, a variety of exposure conditions may be used, depending on the photosensitivity of the drug substance involved and the intensity of the light sources used. For development and validation purposes it is appropriate to limit exposure and end the studies if extensive decomposition occurs. For photostable materials, studies may be terminated after an appropriate exposure level has been used. The design of these experiments is left to the applicant’s discretion although the exposure levels used should be justified.
强制降解试验研究的目的是对药物的总的光敏性进行研究评价,以建立测试方法和说明降解路径。强制降解试验对象包括药物本身和(或)它的简单的溶液或混悬液,以确证分析方法。在这些研究中,样品应盛装在化学惰性的透明容器中。强制降解研究中可能使用各种暴露条件,这取决于药物本身的光敏性和所使用的光源强度。在建立和论证(方法)时,如发生大量分解,即可限制暴露和中止研究。对于光稳定性物质,在适当的光暴露后即可以中止研究。虽然采用的暴露水平是应经论证的,但试验设计可由申报者自己定。
Under forcing conditions, decomposition products may be observed that are unlikely to be formed under the conditions used for confirmatory studies. This information may be useful in developing and validating suitable analytical methods. If in practice it has been demonstrated they are not formed in the confirmatory studies, these degradation products need not be further examined.
在强制条件下,可能会观察到在确认研究条件下不易生成的分解产物,这一信息对建立和论证分析方法是有用的。如果实际证明这些降解产物在确认研究中并不会产生,就不必对它们进一步实验。
Confirmatory studies should then be undertaken to provide the information necessary for handling, packaging, and labeling (see section I.C., Procedure, and II.A., Presentation, for information on the design of these studies).
接着进行确认研究以提供处理、包装、标签等所需要的信息(设计这些研究所需的信息,见1.C方法和IIA样品放置)
Normally, only one batch of drug substance is tested during the development phase, and then the photostability characteristics should be confirmed on a single batch selected as described in the Parent Guideline if the drug is clearly photostable or photolabile. If the results of the confirmatory study are equivocal, testing of up to two additional batches should be conducted. Samples should be selected as described in the Parent Guideline.
一般说来,在研制阶段只需测试一批样品,如果原料药明显光稳定或光不稳定,可以根据指导原则选择一批样品来确定光稳定性特征。如果确认结果不明确,应加试两个批号的样品,样品选择应符合总指导原则。
A. Presentation of Samples 样品的放置
Care should be taken to ensure that the physical characteristics of the samples under test are taken into account and efforts should be made, such as cooling and/or placing the samples in sealed containers, to ensure that the effects of the changes in physical states such as sublimation, evaporation or melting are minimized. All such precautions should be chosen to provide minimal interference with the exposure of samples under test. Possible interactions between the samples and any material used for containers or for general protection of the sample, should also be considered and eliminated wherever not relevant to the test being carried out.
应充分考虑受试样品的物理性质,采取措施如冷藏和&或置密闭容器中,以确保物理状态变化如升华、蒸发、熔化所造成的影响为最小。应采取各种预防措施使供试品光照时少受其他因素的影响。无论与进行的试验有无关系,都应考虑并排除样品与包装材料之间可能存在的相互反应。
As a direct challenge for samples of solid drug substances, an appropriate amount of sample should be taken and placed in a suitable glass or plastic dish and protected with a suitable transparent cover if considered necessary. Solid drug substances should be spread across the container to give a thickness of typically not more than 3 millimeters. Drug substances that are liquids should be exposed in chemically inert and transparent containers.
固体原料药的样品,应取适量放在适宜的玻璃或塑料碟中,必要时以适宜的透明盖子保护。固体药物应分散在容器中,厚度不超过3毫米。液体药物应存放在化学惰性的透明容器中。
B. Analysis of Samples 样品分析
At the end of the exposure period, the samples should be examined for any changes in physical properties (e.g., appearance, clarity, or color of solution) and for assay and degradants by a method suitably validated for products likely to arise from photochemical degradation processes.
光照时间一到,就要检查样品是否有物理性质(如外观、溶液的颜色或澄清度)、含量和降解物的变化。用一种经论证可以检测出光降解物质的合理方法,测定光化反应降解产生的降解物质。
Where solid drug substance samples are involved, sampling should ensure that a representative portion is used in individual tests. Similar sampling considerations, such as homogenization of the entire sample, apply to other materials that may not be homogeneous after exposure. The analysis of the exposed sample should be performed concomitantly with that of any protected samples used as dark controls if these are used in the test.
对于固体原料药,取样应确保在每一项试验中所用的样品具代表性。对于光照后可能会不均匀的物质,取样时需考虑整个样品的均匀化。如果试验中有用于暗度控制研究的样品(可用各种方法将样品保护起来不受光照),则应与光照过的样品同时测定。
C. Judgement of Results 结果判定
The forced degradation studies should be designed to provide suitable information to develop and validate test methods for the confirmatory studies. These test methods should be capable of resolving and detecting photolytic degradants that appear during the confirmatory studies. When evaluating the results of these studies, it is important to recognize that they form part of the stress testing and are not therefore designed to establish qualitative or quantitative limits for change.
“强制降解”研究应设计成能为建立和论证“确认研究”的试验方法提供适当的信息。这些试验方法应能分辨和检测确认研究中出现的光降解物质。在评价这些研究结果时,重要的是应考虑到他们是组成强力破坏试验的一部分,而并不是设计成去建立这些变化的定量和定性限度。
The confirmatory studies should identify precautionary measures needed in manufacturing or in formulation of the drug product, and if light resistant packaging is needed. When evaluating the results of confirmatory studies to determine whether change due to exposure to light is acceptable, it is important to consider the results from other formal stability studies in order to assure that the drug will be within justified limits at time of use (see the relevant ICH Stability and Impurity Guidelines).
确认研究应提供生产和制剂处方中所必要的预防措施,是否需要避光包装。当评估确认研究的结果以决定光照所引起的变化是否可接受时,必须同时考虑其他正式的稳定性研究结果,以确保药物在使用期内符合合理范围。(见ICH 稳定性及杂质指导原则)
3. DRUG PRODUCT制剂药物
Normally, the studies on drug products should be carried out in a sequential manner starting with testing the fully exposed product then progressing as necessary to the product in the immediate pack and then in the marketing pack. Testing should progress until the results demonstrate that the drug product is adequately protected from exposure to light. The drug product should be exposed to the light conditions described under the procedure in section I.C.
通常对制剂的研究应进行一系列试验,首先制剂应完全暴露进行试验,如有必要,再以内包装进行试验,最后以上市包装进行试验。试验一直做到结果证明在光照下药物也受到了足够的保护。制剂应按1.C 章节中所描述的方法进行光照试验。
Normally, only one batch of drug product is tested during the development phase, and then the photostability characteristics should be confirmed on a single batch selected as described in the Parent Guideline if the product is clearly photostable or photolabile. If the results of the confirmatory study are equivocal, testing of up to two additional batches should be conducted.
一般来说,在开发研究阶段,只测试一批样品,如果产品对光稳定或不稳定表现明显,则光稳定试验应按照总指导原则所描述的方法选择一个批号进行;如果确认研究结果比较含糊,应再试验两批以上的样品。
For some products where it has been demonstrated that the immediate pack is completely impenetrable to light, such as aluminium tubes or cans, testing should normally only be conducted on directly exposed drug product.
对有些制剂已证明其内包装完全避光,如铝管或铝罐,一般只需做制剂的直接暴露试验。
It may be appropriate to test certain products such as infusion liquids, dermal creams, etc., to support their photostability in-use. The extent of this testing should depend on and relate to the directions for use, and is left to the applicant’s discretion.
有些制剂如输液、皮肤用霜剂等,应做一些试验证明其使用时的光稳定性。试验的程度取决于使用方式,由申报者自行考虑。
The analytical procedures used should be suitably validated.
所有分析方法应经合适的论证。
A. Presentation of Samples样品的放置
Care should be taken to ensure that the physical characteristics of the samples under test are taken into account and efforts, such as cooling and/or placing the samples in sealed containers, should be made to ensure that the effects of the changes in physical states are minimized, such as sublimation, evaporation, or melting. All such precautions should be chosen to provide a minimal interference with the irradiation of samples under test. Possible interactions between the samples and any material used for containers or for general protection of the sample should also be considered and eliminated wherever not relevant to the test being carried out.
应认真考虑受试样品的物理性质,例如采取措施如冷藏和(或置密闭容器中,以确保物理状态变化如升华、蒸发、熔化所造成的影响为最小。预先应采取必要措施,使受试样品少受这些因素的影响。无论与进行的试验有无关系,都应考虑并排除样品与包装材料之间可能存在的相互反应。
Where practicable when testing samples of the drug product outside of the primary pack, these should be presented in a way similar to the conditions mentioned for the drug substance. The samples should be positioned to provide maximum area of exposure to the light source. For example, tablets, capsules, etc., should be spread in a single layer.
除去包装的受试样品放置条件应与原料药条件相似,保证受到最大面积的光照,如片剂、胶囊剂应分散为单层。
If direct exposure is not practical (e.g., due to oxidation of a product), the sample should be placed in a suitable protective inert transparent container (e.g., quartz).
如果直接暴露不可行(如由于药品易氧化),样品应放在合适的惰性透明容器中(如石英容器)。
If testing of the drug product in the immediate container or as marketed is needed, the samples should be placed horizontally or transversely with respect to the light source, whichever provides for the most uniform exposure of the samples. Some adjustment of testing conditions may have to be made when testing large volume containers (e.g., dispensing packs).
若样品需在内包装或在上市包装的条件下进行试验,样品应水平放置或横面对光源,以保证样品得到最大均匀的光照,当试验大体积容器包装的样品时,有些试验条件应调整(如分散包装)。
B. Analysis of Samples样品的分析
At the end of the exposure period, the samples should be examined for any changes in physical properties (e.g., appearance, clarity or color of solution, dissolution/disintegration for dosage forms such as capsules, etc.) and for assay and degradants by a method suitably validated for products likely to arise from photochemical degradation processes.
光照结束后,应检查样品的所有物理性质(如:外观、溶液的澄清度或颜色、固体制剂如胶囊剂等的溶解度或崩解度)的变化并进行含量、降解产物测定。所采用的分析方法应经过论证,可证实它适合检出光化学降解过程中所生成的物质。
When powder samples are involved, sampling should ensure that a representative portion is used in individual tests. For solid oral dosage form products, testing should be conducted on an appropriately sized composite of, for example, 20 tablets or capsules. Similar sampling considerations, such as homogenization or solubilization of the entire sample, apply to other materials that may not be homogeneous after exposure (e.g., creams, ointments, suspensions, etc.). The analysis of the exposed sample should be performed concomitantly with that of any protected samples used as dark controls if these are used in the test.
对于粉末状样品,取样时应确保每一份测定的供试品具有代表性。对固体口服制剂,应取合适的量,如片剂或胶囊20 片或粒。对光照后可能不均一的样品(如霜剂、软膏、混悬剂)也同样应考虑其采样的代表性问题,如对整个样品进行均匀化或溶解增溶。如果有暗度控制的样品,则光照的样品应与这些保护性样品同时进行分析。
C. Judgement of Results结果判定
Depending on the extent of change special labeling or packaging may be needed to mitigate exposure to light. When evaluating the results of photostability studies to determine whether change due to exposure to light is acceptable, it is important to consider the results obtained from other formal stability studies in order to assure that the product will be within proposed specifications during the shelf life (see the relevant ICH Stability and Impurity Guidelines).
根据变化的程度,可采用特殊标签或包装来限制储藏中的样品的光照。当评估光稳定性试验结果以确定由光照引起何种程度的变化是可接受时,必须综合考虑其他正式稳定性研究的结果,以确保药品在货架寿命产品会一直在规定的规格范围之内。(见ICH稳定性和杂质指导原则)
4. ANNEX附件
A. Quinine Chemical Actinometry
The following provides details of an actinometric procedure for monitoring exposure to a near UV fluorescent lamp (based on FDA/National Institute of Standards and Technology study). For other light sources/actinometric systems, the same approach may be used, but each actinometric system should be calibrated for the light source used.
Prepare a sufficient quantity of a 2 per cent weight/volume aqueous solution of quinine monohydrochloride dihydrate (if necessary, dissolve by heating).
A. 奎宁的光化强度
以下详细介绍监控暴露在近UV, 荧光灯(根据FDA/国家标准和技术研究所)下的光化强度方法。对其他光源/ 光化体系,也可使用相同的方法,但各光化系统均应根据所采用的光源作校正。
准备足量的2%(W/V)盐酸奎宁二水合物的水溶液(必要时加热溶解)。
Option 1
Put 10 milliliters (ml) of the solution into a 20 ml colorless ampoule seal it hermetically, and use this as the sample. Separately, put 10 ml of the solution into a 20 ml colourless ampoule (see note 1), seal it hermetically, wrap in aluminum foil to protect completely from light, and use this as the control. Expose the sample and control to the light source for an appropriate number of hours. After exposure determine the absorbances of the sample (AT) and the control (Ao) at 400 nm using a 1 centimeter (cm) path length. Calculate the change in absorbance, Δ A = AT - Ao. The length of exposure should be sufficient to ensure a change in absorbance of at least 0.9.
方法1
将10ml溶液置20ml无色安瓿中,密封,以此作为样品;另将10ml 溶液置20ml无色安瓿(见注1)密封,包铝箔避光,作为对照;将上述两安瓿于光源中光照数小时后,在400nm波长处,用1cm石英池测定样品吸收度(At)和对照品吸收度(Ao),计算△A=At-Ao。光照时间应足够,确保△A 不小于0.9。
Option 2
Fill a 1 cm quartz cell and use this as the sample. Separately fill a 1 cm quartz cell, wrap in aluminum foil to protect completely from light, and use this as the control. Expose the sample and control to the light source for an appropriate number of hours. After exposure determine the absorbances of the sample (AT) and the control (Ao) at 400 nm. Calculate the change in absorbance, Δ A = AT - Ao. The length of exposure should be sufficient to ensure a change in absorbance of at least 0.5.
Alternative packaging configurations may be used if appropriately validated. Alternative validated chemical actinometers may be used.
方法2:
将溶液加到一个1cm的石英池中,将其作为样品。另外,将溶液加入到一个1cm的石英池中,将其包在铝薄中以保证完全避免光照,将其作为对照样品。在一定的时间段内,将检测样品和对照样品暴露在光照下,光照会影响到样品和对照样品的吸收度,计算二者吸收度的变化,△A=AT-Ao。光照时间应足够,确保△A不小于0.5。
如果经过了验证,其他包装结构也可以使用。可使用其他经论证的光化线测量仪。
Note 1: Shape and Dimensions (See Japanese Industry Standard (JIS) R3512 (1974) for ampoule specifications)
注释一:安瓿的形状和直径(参考日本工业标准(JLS)R3512(1974)中的特定安瓿)
5. GLOSSARY名词解释
Immediate (primary) pack is that constituent of the packaging that is in direct contact with the drug substance or drug product, and includes any appropriate label.
内包装指包装中直接接触原料药或制剂的包装,包括任何适当的标签。
Marketing pack is the combination of immediate pack and other secondary packaging such as a carton.
上市包装指内包装和其他层次包装(如纸盒)的总和。
Forced degradation testing studies are those undertaken to degrade the sample deliberately. These studies, which may be undertaken in the development phase normally on the drug substances, are used to evaluate the overall photosensitivity of the material for method development purposes and/or degradation pathway elucidation.
强制降解试验研究指有意地使样品降解的实验,这些研究一般在原料药处于开发阶段时进行,用于评价药物的总光敏性,以建立测试方法和(或)说明降解途径。
Confirmatory studies are those undertaken to establish photostability characteristics under standardized conditions. These studies are used to identify precautionary measures needed in manufacturing or formulation and whether light resistant packaging and/or special labeling is needed to mitigate exposure to light. For the confirmatory studies, the batch(es) should be selected according to batch selection for long-term and accelerated testings which is described in the Parent Guideline.
确认研究指在标准化条件下确立一个光稳定性特征的实验研究,这些研究用于指导生产工艺是否采取保护性措施,以及决定是否采用避光包装或特使标签来保护药品减少光照。确认研究的样品批号选择应根据总指导原则所规定的长期和加速试验的批号选择。
6. REFERENCES参考书
Quinine Actinometry as a method for calibrating ultraviolet radiation intensity in light-stability testing of pharmaceuticals. 药剂光稳定性测试中用奎宁感光法来校正紫外辐射强度。
Yoshioka S. et al., Drug Development and Industrial Pharmacy, 20 (13), 2049 - 2062 (1994).
Yoshioka S.et al., 药剂研发和工业生产,20(13),2049~2062(1994)
兰贝石药品强光照射试验箱符合以上法规要求,并加入了照度的存储打印功能

 400-600-8767
400-600-8767 13810794957@163.com
13810794957@163.com








